Send us a message
GUIDELINES AND METHODOLOGY
Collecting, handling, and germinating seeds of our native plant species is a relatively straightforward process. There are, not surprisingly, a few challenging species, but the majority can be propagated quite reliably by adhering to a few standard guidelines. These procedures are described in detail in this section, and then briefly cross-referenced as appropriate in the notes for each species.
COLLECTING FRUITS AND SEEDS
When planning to collect native plant seed from anywhere but your own garden, always bear the following in mind:
-
Don't collect on private property or in protected places like parks and conservation areas without prior permission;
-
Never collect seed of plants listed as rare and endangered in your area;
-
Make absolutely sure of the identity of the plants from which you intend to collect, and refer to a field guide, checklist, or complete flora if necessary;
-
Collect only small amounts of fruits/seeds sufficient for your immediate needs, and make sure they are ripe before gathering them;
-
Take care to avoid damaging other parts of the plants from which you are collecting, as well as other nearby plants
-
Fruits/seeds can be gathered in any suitable container; however, in our experience, heavy-duty plastic freezer bags are both the most convenient and reliable, especially if collecting under wet conditions;
-
Immediately label any collected material as to species, location, and collection date;
-
Commence drying or other appropriate fruit/seed handling procedures as soon as possible after collection.

 Normally, fruits should only be collected once they have fully ripened and are entering or about to enter their dispersal phase. The information given in the individual species notes should be used as a guide to the appearance of the fruits/seeds at maturity, and the approximate dates at which this is likely to occur. Local observation and monitoring is, however, an essential added component in deciding precisely when to make a collection.
Normally, fruits should only be collected once they have fully ripened and are entering or about to enter their dispersal phase. The information given in the individual species notes should be used as a guide to the appearance of the fruits/seeds at maturity, and the approximate dates at which this is likely to occur. Local observation and monitoring is, however, an essential added component in deciding precisely when to make a collection.
For most species there is usually a reasonable 'window of opportunity' for making a collection since maturity and dispersal are not reached at exactly the same time by all the fruits on any given plant or by all the plants in a given population; the possible collection window can often be stretched even further by differences in fruit and seed maturity/dispersal dates between separate populations of a given species on different sites.
While it is usually best to wait until the last possible moment before dispersal to make a fruit/seed collection, many species can safely be collected at least several days before the full maturity/dispersal stage is reached (at what we have called the "semi-mature" stage) in order to avoid the risk of missing the seed or having to make a return visit to the collection site. However, there are also some species, mostly ones with fleshy fruits, where the seeds continue to develop and mature within the fruit well beyond the point when the fruit first appears, from its outer colour, to be mature; in these cases, which are all so indicated in the individual species notes, it is very important not to collect until the fruits are starting to drop from the plant.



Finally, if you do not have the opportunity or inclination to collect for yourself, consider contacting a reputable seed house or nursery to enquire about the availability of seeds or plants of the native species of interest. Some are now available commercially, and more may well become so in future if there is enough demand.
HANDLING FRUITS AND SEEDS
DRYING
Many fruits require thorough drying in order to facilitate seed extraction, as well as subsequent seed cleaning and storage. This applies to virtually all so-called "dry fruits" (including most achenes, capsules, cones, follicles, legumes, nuts and nutlets, samaras, schizocarps, and siliques), as well as to a few of the moist fruits (berries, drupes, etc.) where removal of the outer or surrounding fleshy material from the seeds is more easily accomplished if the fleshy material is first allowed to dry and shrivel. The individual species notes indicate all those cases where drying is a necessary and/or convenient first step in the fruit and seed handling process.
Where appropriate, drying is easily accomplished by placing the collected fruits in an open paper (not plastic) bag or envelope, or on an open tray, in a warm, dry location for a few days (or longer if necessary for very wet or fleshy material) until completely dry. Be cautious when drying species with very light (wind-borne) fruits and seeds since any draught or light wind can cause these to be blown around and potentially lost; it is usually advisable to dry such material in a partially open paper bag or envelope rather than on a tray. A similar caution applies to any fruits with sudden and 'explosive' seed dispersal (e.g. Hamamelis spp., Geranium spp., most Lathyrus spp., etc.) which need to be dried in an airy, but more-or-less closed bag or on a tray under a loose cover of paper towel or newspaper so as to prevent the seeds from being ejected all over the place.

.jpg)

SEED EXTRACTION
Procedures for extracting the seeds from collected fruits differ depending on whether the fruits are dry or fleshy, and whether or not they have been subjected to a drying process after collection.
For "Dry Fruits" (most of which will have been subject to a further drying process as described above), there are many species in which all or most of the seeds will have dropped, or been expelled from the fruits during the drying process; in these cases the seeds can simply be gathered from the tray or bag/envelope and placed in a fresh, clean bag or container for temporary or extended storage.
In other species, the seeds may not drop spontaneously from the dry fruits; some of these may only need some additional shaking in a closed bag to cause the seeds to fall out, but others may need more-or-less vigorous rubbing by (gloved) hand in a bowl to forcibly remove the outer layers (bracts, calyx, capsule walls, etc.) from around the seeds.
For certain other species with relatively large dry fruits (most notably many trees and shrubs), the seeds will need to be extracted by hand from individual fruits, for example: from surrounding 'husks' (e.g. Carya, Juglans, Aesculus), from acorn 'cups' (Quercus), from legumes/pods (e.g. Gymnocladus, Gleditsia), or from follicles (e.g. Magnolia).
Finally, it should be noted that seeds of certain species are relatively soft and easily damaged, and should not be removed from the protective outer layer(s) of the fruit (e.g. Desmodium spp., Hylodesmum spp., Dirca palustris, as well as most species, such as Acer, Fraxinus, and Ulmus, whose fruits are samaras).
Necessary and/or recommended seed extraction procedures are given in the notes for each individual species.


.jpg)
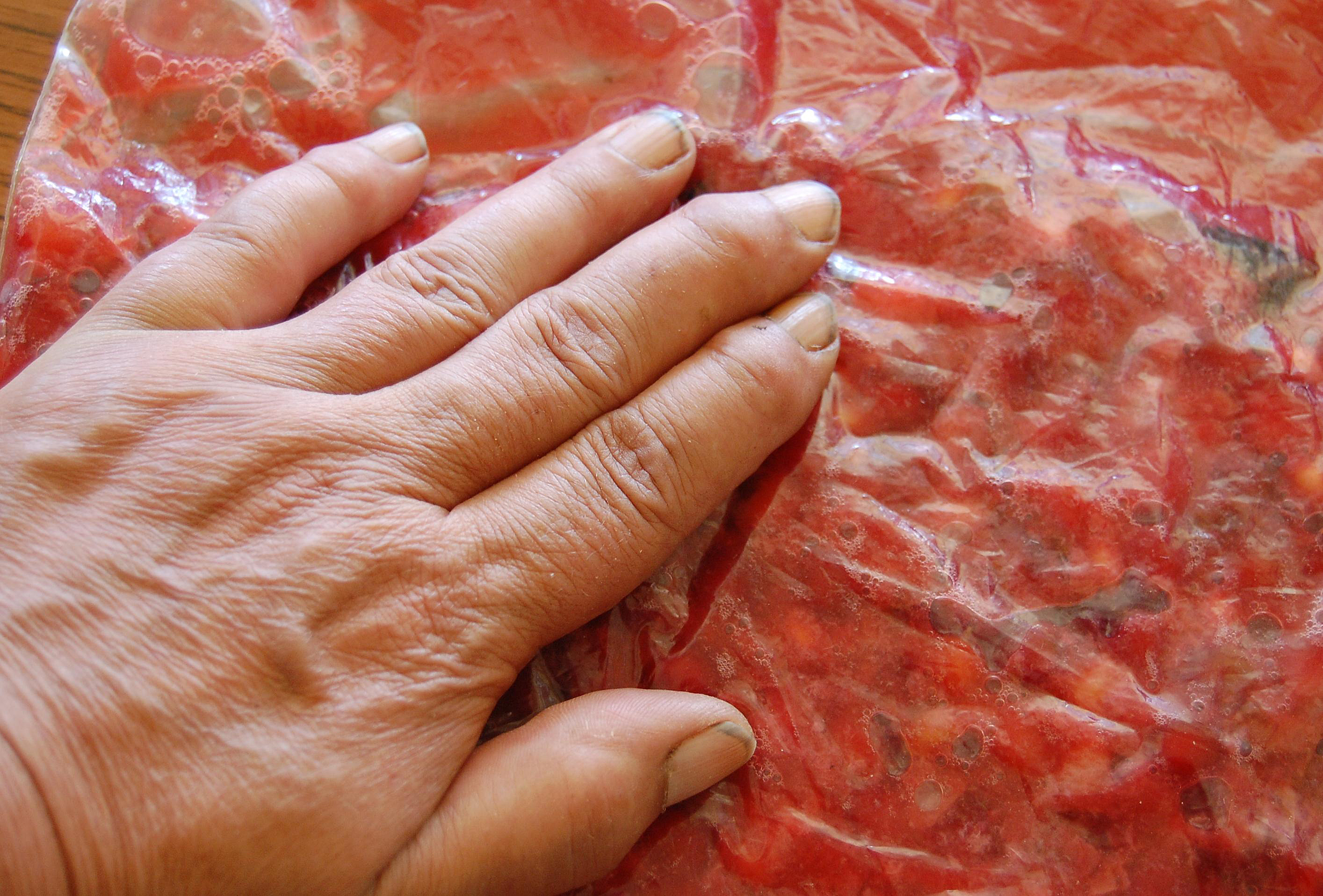
For "Fleshy Fruits" there are 3 main approaches to seed extraction, preceded by the following general rule: EVERY fleshy fruit contains a germination inhibitor (in the flesh of the fruit) which must, in every case, be removed, and the remaining seed(s) washed with soap and water, before applying any germination treatment(s). In nature, this “cleaning” is normally performed by the combination of environmental factors to which the fruits are exposed. If the seeds of fleshy fruits are not cleaned in this manner (and/or you choose to sow them directly outside), then a prolonged germination period can be expected.
The majority of fleshy fruits will have originally been collected in a strong plastic bag. Many of these can be left in the plastic bag until the fleshy layer has fully softened. Then the fruit is squashed by hand through the bag so as to separate the seeds (or pyrenes) from the surrounding fleshy material. The entire resultant mushy mess (!) is then placed into a bowl of water (which may assist in further separating any remaining seeds from fleshy material). At this stage, the debris may float and can be carefully poured out (with the seeds normally remaining on the bottom of the bowl). Some small seeds can be pushed against a sieve over a bowl and the extracted seeds washed through. Normally a combination of different sized sieves and bowls will work. For fruits that have an outer fleshy layer enclosing a hard inner seed wall (endocarp), no attempt should be made to break the protective endocarp wall to reveal the actual seed(s) inside.
For fruits with a fleshy outer layer enclosing multiple seeds that are surrounded by, and often largely separated from, an amount of internal fleshy or pithy material (includes some berries and capsules), it is quite easy and straightforward to simply push the seeds out by hand from within each fruit, followed by washing (e.g. Trillium, Asarum, etc).
In the case of fleshy fruits where it is preferable to subject the fruit to a drying process following collection, it is necessary to rub the fruits after drying to remove the (now dry) fleshy material from the seeds, or, alternatively, to pick the seeds out by hand from among the surrounding material. The extracted seeds will then still have to be washed (e.g. Epigaea, Arisaema, etc).
The seed extraction procedures required and/or most appropriate for each species are given in the individual species notes.
.jpg)



SEED CLEANING
 Seed cleaning is an important part of the overall seed handling process when preparing large quantities of seed, especially for commercial purposes. It is much less important when dealing with small batches for personal use, but there are still a few steps that are often worth taking for convenience and other reasons in relation to subsequent seed storage and germination.
Seed cleaning is an important part of the overall seed handling process when preparing large quantities of seed, especially for commercial purposes. It is much less important when dealing with small batches for personal use, but there are still a few steps that are often worth taking for convenience and other reasons in relation to subsequent seed storage and germination.
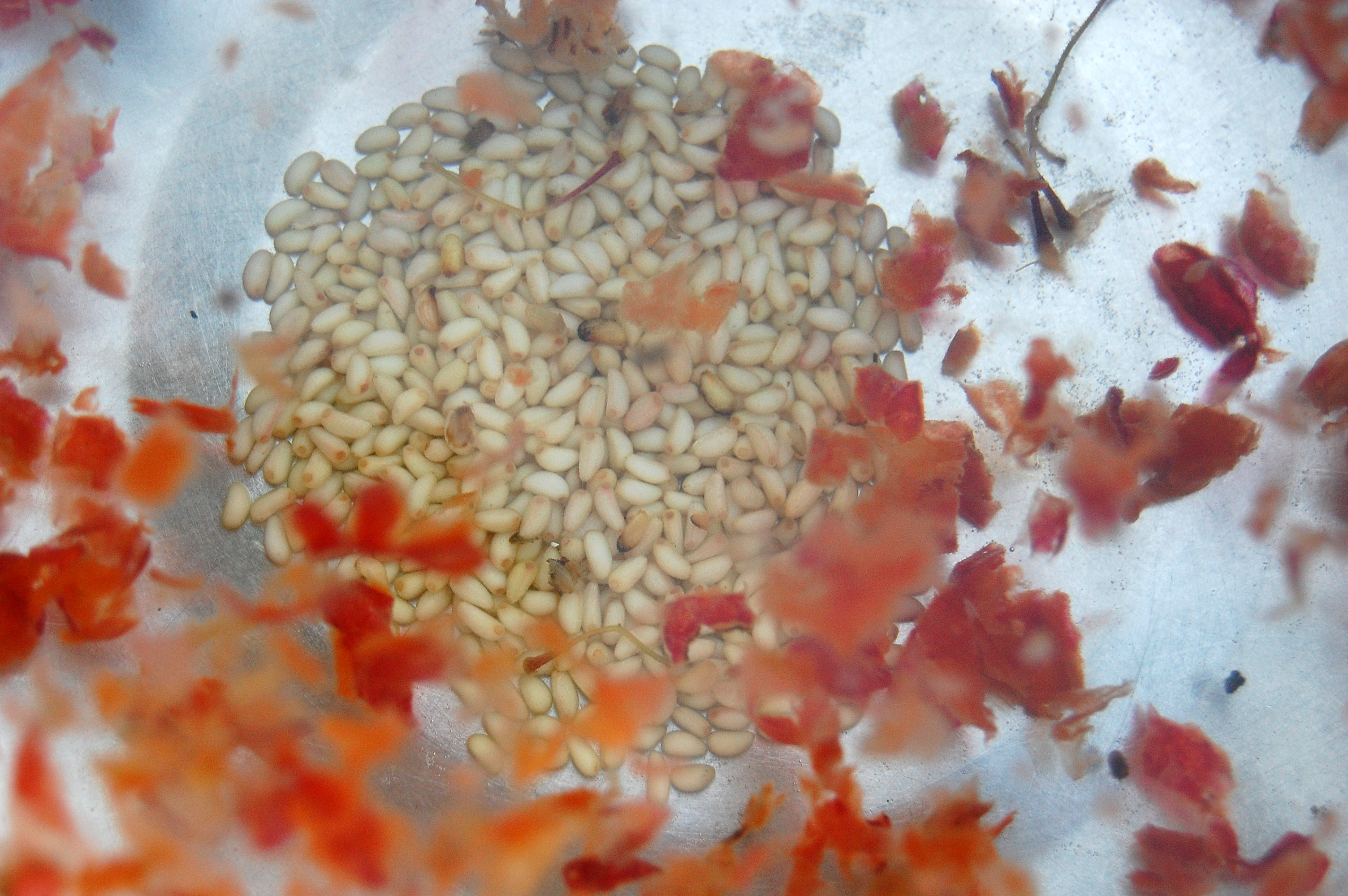
It is always helpful to remove as much debris as possible from batches of seed so as to have a better appreciation of the actual quantity and quality of seed that has been obtained; this can be done by any appropriate combination of removing large pieces of debris by hand, and sieving and/or winnowing of the material. This is also an appropriate stage at which to remove any seeds that appear insect-damaged, obviously discoloured, malformed, or otherwise not fully developed.
It may also greatly facilitate subsequent storage and/or germination treatments if most unnecessary attachments, such as feathery plumes, pappus hairs, or large wings of samaras, are at least partially removed by gentle rubbing.
Finally, it is important, as already noted above, that as much as possible of the outer and/or surrounding fleshy material be removed from the seeds of plants with fleshy fruits so as to ensure that fungal attack does not set in during storage and that any germination inhibitors in the flesh of the fruit are removed before attempting to germinate the seeds.
Any instances where seed cleaning is particularly important or helpful are noted in the individual species notes.
SEED STORAGE
After necessary preparation, seeds of most of our native species can be stored for lengths of time ranging from a few days or weeks up to several years, depending on the species and the storage conditions. In this respect there are 2 broad classes of seeds - those that can be stored dry, and those that must be kept moist in order to retain their viability.

 Many of our native species have seeds that can withstand dry storage, either at ambient temperatures or in a freezer. For all these species, the seeds should be placed in small paper bags or glassine envelopes, labelled with their species name, origin, and storage date, and then placed in a dry, dark storage place at ambient temperature or into a freezer. The length of time that most seeds retain their viability varies widely between species, and is almost always longer in a freezer than at ambient temperatures. Plants whose seeds retain their viability for only a short time in dry storage are all identified in the individual species notes.
Many of our native species have seeds that can withstand dry storage, either at ambient temperatures or in a freezer. For all these species, the seeds should be placed in small paper bags or glassine envelopes, labelled with their species name, origin, and storage date, and then placed in a dry, dark storage place at ambient temperature or into a freezer. The length of time that most seeds retain their viability varies widely between species, and is almost always longer in a freezer than at ambient temperatures. Plants whose seeds retain their viability for only a short time in dry storage are all identified in the individual species notes.
Some of our native species cannot withstand any extended periods of drying and must be stored in moist conditions if they are to remain alive for more than a few days after extraction from the fruits. Seeds of these species must be given an appropriate germination treatment immediately after extraction or otherwise placed into moist (but not overly wet) vermiculite in plastic bag(s) without delay. If you do this, avoid the use of heavy-duty freezer bags as they do not allow sufficient oxygen transfer necessary for survival of the seeds; it is best to use thinner plasic 'storage' or 'lunch' bags. If stored moist in this way, these seeds can be expected to retain their viability for at least a few months; they are, however, never long-lived in the same way as many 'dry storage' species. Plants whose seeds must be treated in this way are all clearly identified in the individual species notes.
GERMINATION PROTOCOLS
Successful germination is the intended culmination of all the preceding seed collection and handling procedures. While it may seem obvious, it nevertheless bears stressing that properly conducted collection and handling procedures are an essential pre-requisite for any and all germination treatments; dead or dying seeds have never been known to germinate well!
Each of our native species has a requirement for a particular condition (=treatment) or combination of conditions to stimulate germination. For some species this may be as simple as sufficient warmth and moisture at any time after seed ripening, while others require an initial cold period followed by warmth, or a more complicated and longer pattern of alternating cold and warm conditions. In addition, some seeds require a sufficient minimum level of light before they will germinate, while yet others require, or at least benefit significantly from, the presence of gibberellins (naturally-occurring, growth-promoting substances in the soil; more about these later). The most appropriate germination treatment for a given species may also differ depending on whether its seeds are fresh or have been stored dry for some time. Finally, there are some native species (including all the orchids) that need to form an immediate association with soil mycorrhizae, or to encounter the presence of other, as yet not well understood, organisms and/or their biochemical products in the soil, before they are able to germinate.
The necessary (or alternative) germination protocol(s) to follow for each species are provided in the individual species notes. A general description of each of the possible germination requirements is given below, followed by information on a number of other issues or considerations related to the overall germination and early seedling development process.
-
Warmth: All our native species require sufficient warmth to initiate and/or complete the germination process, regardless of whatever other treatments they may need. In this context, "warm" conditions are usually understood to mean 15 to 25 degrees C. in a controlled environment, or ambient (spring/summer) temperatures that reach into this range in the garden.
-
Moisture: Along with adequate warmth, this is the other universally-required ingredient for successful germination of all our native species. For many species (those that can safely be stored dry), moisture is only necessary at the time the appropriate seed germination treatment is started, regardless of whether this begins with a warm or a cold period. However, for a number of others, adequate moisture is a constant need after their seed has been collected and cleaned, and their seeds will soon die if allowed to dry out for more than a very short time; seeds of these species are usually relatively short-lived, and they must be kept moist until the appropriate seed germination treatment is started. Adequate moisture is hard to define in numeric terms, but it signifies a consistent, moist, humid environment in the seed sowing medium, sufficient to maintain a film of moisture on the surface of the seeds but not so wet as to create waterlogged conditions (which will deprive the seeds of an adequate oxygen supply and kill them if sustained for long).
-
Cold: For many of our native species, a period of (moist) cold is necessary, before moving to warm, in order to stimulate germination. The cold period allows seeds to complete their maturation and/or undergo necessary internal biochemical/physiological changes essential for germination; in the wild it also serves to keep germination in synchrony with the natural seasonal cycle. A "standard cold treatment" is normally considered to be a period of at least 3 months in which the seed is kept between -4 and +4 degrees C., in moist vermiculite in a plastic bag, or in moist soil-less seeding mix in a pot that is in turn within a plastic bag, and stored in a refrigerator or cold storage area. In areas with climates similar to our region, the same cold treatment can usually be obtained by sowing the seeds in moist seeding mix in pots or flats and leaving them outside at ambient temperatures through the winter months; if using this approach, it is best to plunge the pots into a location with freely-drained (sandy) soil so as to prevent waterlogging and/or exposure to excessively cold air temperatures. Finally, it is critically important to remember that a cold treatment always requires a moist condition; subjecting dry seeds to a cold period will have absolutely no effect on germination.
-
Light: Provided all of their other germination requirements are met, the seeds of many of our native species will commence germination in dark or very low light conditions. They do, of course, need adequate ambient light levels as soon as the young shoots and leaves begin to appear, but light is not an essential initial trigger for the germination process. This is not the case, however, for some of our species, including many aquatic and other water-loving plants. For these species, in addition to their other germination requirements, the seeds must receive a sufficient minimum intensity of light for germination to occur. This can normally be achieved by sowing the seed outdoors at ambient light levels, or indoors in a bright/sunny window or under grow lights; it is also important to not cover the seeds of these species with the growing medium when sowing them.
-
Gibberellins: These are naturally-occurring substances, produced by various soil-dwelling fungi, that are found to a greater or lesser extent in virtually all of our native soils. Several dozen different gibberellins have now been isolated, any number of which may have a significant (usually beneficial but occasionally detrimental) effect on the germination and early seedling development of one or other of our native plant species. The most ubiquitous gibberellin, and the only one that is readily available commercially, is known as GA3; this compound has been shown to have a significant beneficial effect on the germination of a substantial number of our native species (but also a detrimental effect on a few others), especially in terms of increased speed of germination and/or raised percentage germination rates. GA3 is probably only rarely (if ever?) an absolute requirement to achieve germination, but in some species it may obviate the need for one or more periods of cold treatment and/or improve the probability of obtaining reasonable levels of germination within a predictable time frame.
-
Response Times and Scheduling of Germination Treatments: The length of time that it takes seeds to respond to their 'final' warm-moist treatment (whether this is the only treatment they require or the last one in any cold-warm series) varies enormously between species. For some, the seeds may begin germinating in as little as 5 or 6 days, while others, especially some trees and shrubs, may need 2 or 3 months or more. Total time elapsed from initial seed sowing to the appearance of germinants differs even more widely, from a total of less than a week, up to at least 2 or 3 years for some species that require a series of repeated cold and warm treatments. If seeds are sown directly in the garden, or in pots/flats that are then placed in the garden to receive their 'germination treatments' directly from the natural seasonal changes, then these are the total times that must pass before any germination will occur. If, instead, seeds are sown in a heated greenhouse, or indoors in a bright window or under grow lights (with alternating cold periods as needed in a refrigerator or similar), then some reduction of the total time required can often be achieved; however, careful scheduling is usually necessary so as to ensure that the resultant seedlings are large enough, sufficiently acclimatized ("hardened off"), and ready to transplant outside at an appropriate time of year. For a lot of our native species, patience is an essential component of their germination protocols!
-
Germination Media: There are essentially 2 choices for suitable germination media: vermiculite or a similar sterile, moisture-retentive substance, usually placed in a strong plastic bag; or a good quality, sterilized, soil-less seeding mix, usually placed in a pot or flat. Vermiculite in plastic bags is often the easiest to use (especially when cold periods in a refrigerator are planned), but it offers restricted growing space and contains no nutrients, so the emergent seedlings must be transplanted into a suitable growing medium soon after emergence; it is therefore best suited for moderate- to large-sized seeds/seedlings which can be handled safely at an early stage. Sterilized seeding mix in a pot or flat is often the better choice for small seeds/seedlings which can be left to grow (and thinned if necessary) in situ until they are big enough to transplant. Of course, directly sowing the seeds into an apparently suitable site in the garden is always available as a potential third option; while undoubtedly the simplest, it is usually also the riskiest since it subjects the seeds to much greater variation in seedbed condition and to higher likelihood of fungal and insect attack and predation by birds and rodents. Germination (and subsequent seedling survival) rates are almost always substantially lower for directly sown seeds as compared to those sown under more controlled conditions.
-
Species with Erratic, Unpredictable, Extended or Unknown Germination Patterns and Requirements: Despite attempts by many people, there remain a significant number of native species for which it is still at the present time impossible to specify any effective and reliable germination protocols. For some of these, such as the orchids, the difficulties appear to centre around the need for the seeds to form an immediate symbiotic association with particular soil mycorrhizae, a "treatment" currently impossible to apply under controlled conditions. For some others, with an erratic, often much-extended, and apparently unpredictable germination pattern, the "problem" may not lie in the germination treatments themselves but rather in the variable genetic make-up of the seeds which have evolved to respond to similar (or changing) environmental conditions in different ways, as a long-term survival mechanism for the species; with sufficient patience it is often possible to obtain a few germinants of species like this, but at (seemingly) unpredictable intervals. Work is ongoing on many of these "difficult" species, but at least for now there remain a number for which our recommendation of an appropriate germination protocol has to be: "Sow the seeds directly on an apparently suitable site in the garden and hope for the best!". (And please let us know if you discover an approach that seems to work!). Finally, bear in mind, also, that an occasional unexplained failure to germinate in a given batch of seed is an inevitable part of the challenge of dealing with biological systems; even apparently healthy seeds of a species with reliable, well-established germination protocols can sometimes fail because of unseen attack from pathogens or developmental issues resulting from environmental stress.








.jpg)

SPECIAL INFORMATION REGARDING FERNS AND ORCHIDS
Different procedures are required for handling and germinating ferns, which reproduce by means of spores rather than seeds; details can be found in the section dealing specifically with this group of plants.
Orchids also present many difficulties for propagation from seed, and there are at present no well-established handling or germination protocols for the species that occur in our region; further details are given in the section dealing with this group.
GLOSSARY OF TYPES OF FRUITS
Note: Larger versions of the accompanying photos can be found on the individual species pages.
-
Achene: A simple, dry, indehiscent fruit that develops from a single ovary and lacks the specialized features that characterize a caryopsis, nut, samara, or utricle; usually single-seeded; seed(s) not released from the fruit at maturity. Typical examples: Symphyotrichum (Aster) spp.; Carex spp.; Solidago spp..
 (Bidens cernua)
(Bidens cernua) -
Aggregate Fruit: A fruit derived from 2 or more pistils of a single flower; the basic fruit type may vary, for example an aggregate of achenes or an aggregate of drupelets. An aggregate fruit that contains some other flower parts in addition to the pistils, for example a fleshy receptacle, as an integral part of the mature fruit is referred to as an aggregate accessory. Typical examples: Ranunculus spp. (aggregate of achenes); Rubus spp. (aggregate of drupelets); Aquilegia canadensis (aggregate of follicles).
 (Ranunculus fascicularis)
(Ranunculus fascicularis) -
Berry: The most general type of fleshy fruit, derived from a single pistil, in which the entire fruit wall is fleshy; usually contains several to many seeds embedded within the flesh. Typical examples: Trillium spp.; Vaccinium spp.; Lonicera spp..
 (Ribes hirtellum)
(Ribes hirtellum) -
Capsule: A dry, dehiscent fruit formed from a compound ovary (i.e. from more than one carpel); opens at maturity to release the seeds through slits, pores, or by splitting into valves. Typical examples: Lilium spp.; Lobelia spp.; Viola spp..
 (Viola labradorica)
(Viola labradorica) -
Caryopsis: A fruit of a member of the grass family (Poaceae), typically dry and indehiscent, in which the seed coat of the single seed is attached to or grown together with the fruit wall (pericarp). Typical examples: Bromus spp.; Deschampsia spp.; and all other true grasses.
 (Andropogon gerardii)
(Andropogon gerardii) -
"Cleistogamous Fruit": This term refers to a process in which the flowers do not open (and are often without normal petals) and are self-fertile, producing fruits that appear essentially the same as those resulting from open pollination, but that are exact genetic replicas of the parent plant. In principle, these so-called "cleistogamous fruits" can belong to any one of the types of fruits described in this glossary; in practice, however, most are either capsules or legumes.
 (Polygala polygama)
(Polygala polygama) -
Cone: A reproductive structure typical of conifers (Order Pinales), composed of a series of overlapping pollen- or ovule-bearing scales arranged around a central axis. Male (pollen-bearing) cones usually remain fleshy while female (ovule-bearing) cones usually become woody at maturity. The true fruits of cone-bearing species are the individual scales with their attached naked seeds; however, the complete cones are often referred to as 'fruits' in a more general sense. Typical examples: Pinus spp.; Picea spp.; Thuja spp..
 (Tsuga canadensis)
(Tsuga canadensis) -
Drupe: A fruit with a fleshy outer fruit wall (exocarp) and a hard inner fruit wall (endocarp) enclosing one or more seeds. Typical examples: Cornus spp.; Prunus spp.; Viburnum spp..
 (Prunus americana)
(Prunus americana) -
Drupelet: A small drupe. Typical examples: Rubus spp..
 (Rubus pubescens)
(Rubus pubescens) -
Follicle: A dry, dehiscent fruit formed from a simple ovary (single carpel) which splits open on one side along the seed-bearing suture at maturity to release the seeds. Typical examples: Asclepias spp.; Magnolia spp.; Spiraea spp..
 (Asclepias incarnata)
(Asclepias incarnata) -
Legume: A fruit of a member of the Fabaceae, Caesalpinaceae, or Mimosaceae family, formed from a simple ovary (single carpel); usually dry, several-seeded, and splitting open along both sutures at maturity to release the seeds. Typical examples: Desmodium spp.; Lathyrus spp.; Gymnocladus dioicus.
 (Lathyrus ochroleucus)
(Lathyrus ochroleucus) -
Multiple Fruit: A fruit derived from several to many flowers. Typical examples: Morus spp.; Symplocarpus foetidus.
 (Morus rubra)
(Morus rubra) -
Nut: A dry, indehiscent fruit developed from a compound ovary; hard-walled and usually containing a single seed. Typical examples: Corylus spp.; Juglans spp.; Quercus spp..
 (Corylus cornuta)
(Corylus cornuta) -
Nutlet: A small nut; can also be considered a hard-walled achene. Typical examples: Betula spp.; Mertensia spp.; Carpinus caroliniana.
 (Carpinus caroliniana)
(Carpinus caroliniana) -
Pod: A general term for any kind of dry, dehiscent fruit. Often used interchangeably with 'Legume' in describing fruits of the Fabaceae.
 (Cercis canadensis)
(Cercis canadensis) -
Pome: A fruit derived from a compound ovary, with a fleshy outer fruit wall (exocarp) enclosing a 'core' in which there are usually several seeds. Typical examples: Amelanchier spp.; Crataegus spp.; Sorbus spp..
 (Sorbus decora)
(Sorbus decora) -
Pyrene: A small bony unit containing a single seed, derived from the endocarp of a drupe or pome; such a fruit usually has two or more pyrenes (also sometimes referred to as 'nutlets'). Typical examples: Panax spp.; Arctostaphylos uva-ursi.
 (Panax quinquefolius)
(Panax quinquefolius) -
Samara: A winged, indehiscent fruit; commonly referred to as a 'key'. Typical examples: Acer spp.; Fraxinus spp.; Ulmus spp..
 (Acer pensylvanicum)
(Acer pensylvanicum) -
Schizocarp: A dry, dehiscent fruit formed from a compound ovary, which splits into separate, one-seeded carpels (mericarps) at maturity. Typical examples: Verbena spp.; Monarda spp.; Geranium maculatum.
 (Monarda didyma)
(Monarda didyma) -
Silique: An elongated capsule in which the two valves are shed at maturity from the central membrane (replum) to which the seeds are attached. These are the characteristic fruits of the family Brassicaceae. Typical example: Cardamine spp..
 (Cardamine diphylla)
(Cardamine diphylla) -
Sporangia; Sori: Fruiting structures of ferns. See section on 'Ferns' for further details.
 (Dryopteris cristata)
(Dryopteris cristata) -
Utricle: A small, indehiscent, thin-walled, single-seeded, more-or-less inflated fruit; in effect, an inflated achene. Typical example: Pontederia cordata.
 (Pontederia cordata)
(Pontederia cordata)
SOME USEFUL RESOURCES CONCERNING NATIVE PLANT IDENTIFICATION AND PROPAGATION, AND SPECIES CHECKLISTS FOR EASTERN CANADA
Printed Publications:
-
Anon., 2001. Canada: Plant Hardiness Zones (Fold-out Map). Government of Canada, Natural Resources Canada and Agriculture and Agri-food Canada.
-
Argus, G.W., K.M. Pryer, D.J. White and C.J. Keddy. 1982-87. Atlas of the Rare Vascular Plants of Ontario. Parts 1-4. National Museum of Natural Sciences (now Canadian Museum of Nature), Ottawa, ON.
-
Chambers, B., K. Legasy and C.V. Bentley. 1996. Forest Plants of Central Ontario. Lone Pine Publishing, Edmonton, AB.
-
Cobb, B., E. Farnsworth and C. Lowe. 2005. A Field Guide to Ferns and their Related Families: Northeastern and Central North America. 2nd Ed. Houghton Mifflin Co., Boston & New York.
-
Crowder, A., K.E.J. Topping and J.C. Topping. 1997. Plants of the Kingston Region. The Fowler Herbarium, Dept. of Biology, Queen's University, Kingston, ON.
-
Deno, N. Seed Germination Theory and Practice. Original Publication plus 2 Supplements. (Available direct from the author : Dr. Norman Deno, 139 Lenor Drive, State College, PA 16801, USA).
-
Dickinson, T., D. Metsger, J. Bull and R. Dickinson. 2004. The ROM Field Guide to Wildflowers of Ontario. Royal Ontario Museum and McClelland & Stewart Ltd., Toronto, ON.
-
Farrar, J.L. 1995. Trees in Canada. Fitzhenry & Whiteside Ltd. and Canadian Forest Service, Natural Resources Canada.
-
Gillett, J.M. and D.J. White. 1978. Checklist of Vascular Plants of the Ottawa-Hull Region, Canada. National Museum of Natural Sciences (now Canadian Museum of Nature), Ottawa, ON.
-
Gleason, H.A. and A. Cronquist. 1991. Manual of Vascular Plants of Northeastern United States and Adjacent Canada. 2nd Ed.. The New York Botanical Garden Press, Bronx, NY.
-
Hinds, H.R. 1986. Flora of New Brunswick. Primrose Press, Fredericton, NB.
-
Holmgren, N.H. 1998. Illustrated Companion to Gleason and Cronquist`s Manual: Illustrations of the Vascular Plants of Northeastern United States and Adjacent Canada. The New York Botanical Garden Press, Bronx, NY.
-
Kershaw, L. 2001. Trees of Ontario, including Tall Shrubs. Lone Pine Publishing, Edmonton, AB.
-
Marie-Victorin, Fr.. 1964. Flore Laurentienne. 2nd Ed. Les Presses de l`Universite de Montreal, Montreal, QC.
-
Morton, J.K. and J.M. Venn. 2000. The Flora of Manitoulin Island. 3rd Ed. Department of Biology, University of Waterloo, Waterloo, ON.
-
Newmaster, S.G., A.G. Harris and L.J. Kershaw. 1997. Wetland Plants of Ontario. Lone Pine Publishing, Edmonton, AB.
-
Peterson, R.T. and M. McKenny. 1968. A Field Guide to Wildflowers: Northeastern and North-Central North America. Houghton Mifflin Co., Boston & New York.
-
Reddoch, J.M. and A. Reddoch. 1997. The Orchids in the Ottawa District: Floristics, Phytogeography, Population Studies and Historical Review. In: The Canadian Field Naturalist, Vol. 111(1), 1-186.
-
Soper, J.H. and M.L. Heimburger. 1985. Shrubs of Ontario. 2nd Ed.. The Royal Ontario Museum, Toronto, ON.
-
Zink, M. 1998. Roland`s Flora of Nova Scotia. 2 vols.. Nova Scotia Museum and Nimbus Publishing.
On-line Resources:
-
Brouillet L, Desmet P, Coursol F, Meades S J, Favreau M, Anions M, Belisle P, Gendreau C, Shorthouse D, and contributors (2010 +). Database of Vascular Plants of Canada (VASCAN). Online at http://data.canadensys.net/vascan
-
Coulson, D. Checklist of the Flora of Renfrew County (Ontario). Available at Coulson (2020) Renfrew County, Ontario.pdf
-
Meades, S.J. and Meades, W.J. 2021. Flora of Newfoundland and Labrador. Online at https://newfoundland-labradorflora.ca
-
White D J. 2016. Plants of Lanark County, Ontario - 2016 Edition. Online at www.lanarkflora.com